I am a physician-scientist with expertise in biomedical engineering, multiomics, and artificial intelligence. I am currently a cardiology fellow at Johns Hopkins Hospital. I obtained my PhD in biomedical engineering from Johns Hopkins University, MD from Harvard Medical School, and internal medicine residency from NYU Langone Health & Bellevue Hospital. My interests are in improving cardiometabolic health and obesity through scientific discoveries, clinical care, and community outreach. I am now studying the genetic underpinnings, environmental impacts, and cardiovascular implications of clinical obesity.
Experience
- Postdoctoral Research Fellow with Dr. Alexis Battle and Michael Blaha at Johns Hopkins University (2025-Present)
- Cardiology Fellow at Johns Hopkins Hospital (2024-Present)
- Medicine Resident Physician at NYU Langone Health/Bellevue Hospital (2021-2024)
- Postdoctoral Research Fellow with Dr. Sean Heffron at NYU Langone Health (2022-2024)
- Engineering Consultant at SpectraWave Inc (2020-2021)
- Postdoctoral Research Fellow with Dr. Sekar Kathiresan at the Broad Institute (2018-2019)
- PhD Student with Dr. Natalia Trayanova at Johns Hopkins University (2013-2017)
- Founder of Archon Medical Technologies (2012-2013)
Research
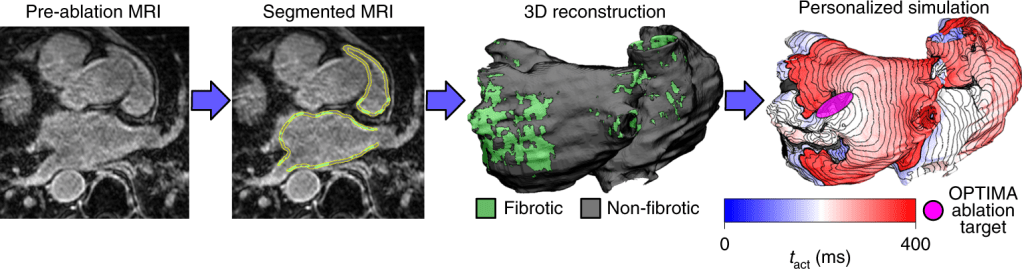
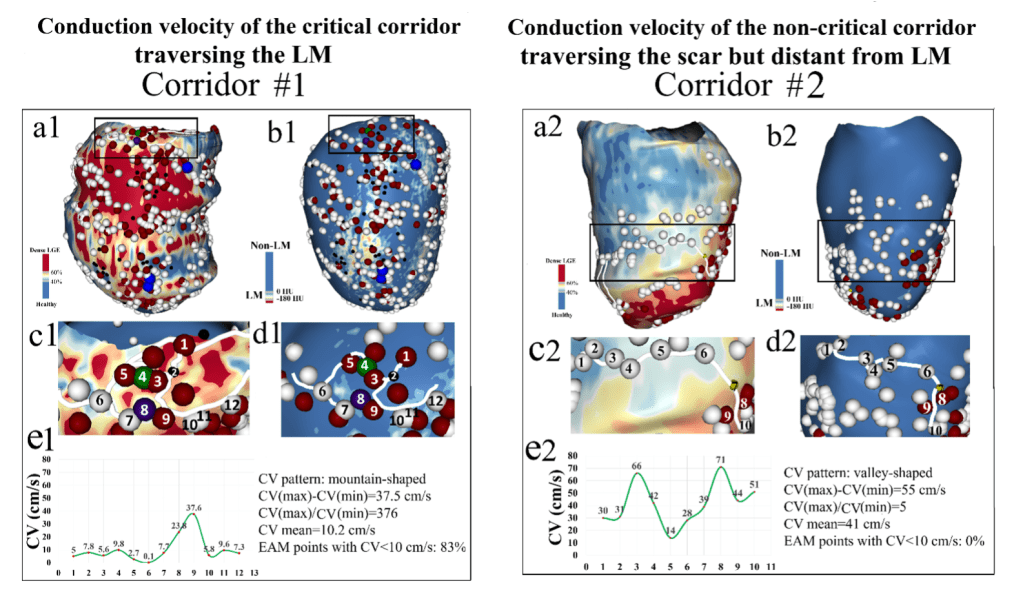
Patient-Specific Models of Atrial Fibrillation
Atrial fibrillation is the most common heart rhythm disorder and is a major contributor to stroke and heart failure. We created personalized computational models using MRI, machine learning, and electrophysiology to predict where atrial fibrillation originates and where clinicians should apply treatment. We are conducting a large clinical trial (OPTIMA AF) to test if this approach can improve patient outcomes.
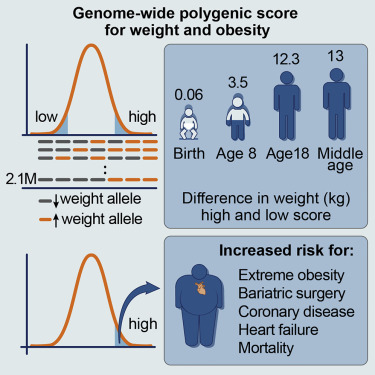
Genetic Risk of Obesity and Cardiovascular Disease
Severe obesity is becoming a global health crisis. We developed a genetic risk score to help determine an individual’s risk for developing severe obesity. We found that people with this genetic risk develop accelerated weight gain in early childhood and have increased risk for cardiovascular disease by middle age.
Fat in Life-Threatening Arrhythmias
Patients with previous heart attacks are prone to life-threatening arrhythmias. Patients with heart attacks also develop lipomatous metaplasia, which is fat that replaces normal heart tissue. We have found that this type of fat changes the normal electrical properties of the heart and increases the risk for life-threatening arrhythmias.
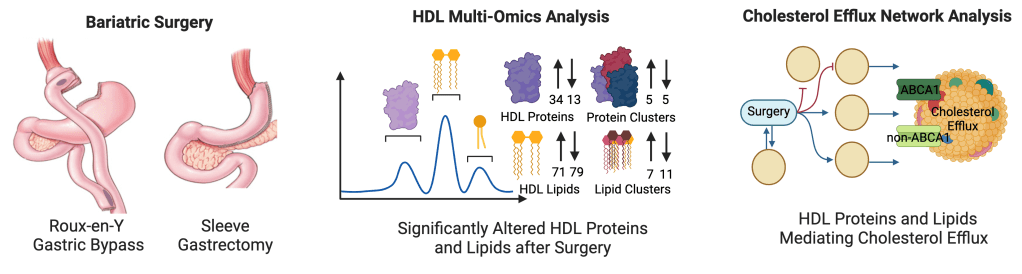
Bariatric Surgery on Lipid Health
Patients who receive bariatric surgery have improved cardiovascular health, thought to be from weight loss, improved HDL, decreased blood pressure, and reduced blood sugar. We found that bariatric surgery transforms the HDL proteome and lipidome to improve cholesterol efflux and overall cardiovascular health.
Publications
Please visit Google Scholar or PubMed to learn more about my work.